SPOILER ALERT!
Why Everyone Is Dead Inappropriate About Hydrochloric Acid Reacts With Caustic Soda And Why You Need To Read This Record
It additionally dissolves many metals (not nickel or its alloys, gold, platinum, or silver), and most plastics. Fluorocarbons corresponding to Teflon (TFE and FEP), chlorosulfonated polyethylenene, pure rubber and neoprene all are proof against hydrofluoric acid. Hydrofluoric acid is so corrosive as a result of the fluorine ion is extremely reactive. Even so, it isn't thought of a 'sturdy' acid because it does not utterly dissociate in water. The metals under hydrogen don't react with dilute acids.
The time period conjugate comes from the Latin stems that means "joined collectively" and refers to things which might be joined, particularly in pairs, corresponding to Brnsted acids and bases. Personal protecting gear corresponding to latex gloves, protecting eye goggles, and chemical-resistant clothes and sneakers will decrease risks when handling hydrochloric acid. The United States Environmental Protection Agency charges and regulates hydrochloric acid as a poisonous substance. Ion exchangers and demineralized water are used in all chemical industries, consuming water manufacturing, and plenty of food industries. (iron(III) chloride from magnetite)Both iron(III) chloride and PAC are used as flocculation and coagulation agents in sewage treatment, drinking water production, and paper production.
Pour about 15 mL of this dilute common indicator resolution right into a clear cup for each scholar group. When https://www.file-upload.com/8iqznq19i40j of a solution is lower than 7, the answer is acidic. The range of concentrations of the H3O+and OH- ions in aqueous resolution is so large that it's difficult to work with. P. L. Sorenson suggested caustic soda pearls 99 msds reporting the concentration of the H3O+ion on a logarithmic scale, which he named the pH scale. Because the H3O+ ion concentration in water is sort of at all times smaller than 1, the log of those concentrations is a adverse number.
Superacids generally are used to synthesize and characterize carbocations. Rapidly and explosively decomposes upon contact with water. Because of this property, fluoroantimonic acid cannot be utilized in aqueous resolution.
H2O, OH-, HSO4-, and NH3, for instance, may be found in both columns within the desk above. Water is the right example of this conduct caustic soda for sale near me because it simultaneously acts as an acid and a base when it varieties the H3O+ and OH- ions.
But this time, the stronger acid and the stronger base are on the best aspect of the equation. The worth of Kafor an acid can be utilized to determine whether it's a strong acid or a weak acid, in an absolute sense. It can be used l to match the relative strengths of a pair of acids. Ka can therefore be used to distinguish between sturdy acids and weak acids. The relative strengths of acids is often described in terms of an acid-dissociation equilibrium fixed, Ka.
Use your graduated cylinder to add 5 mL of water to the cup labeled sodium carbonate. Distribute the cups with common sodium hydroxide suppliers indicator resolution to each student group.
For instance, the acid can be used to take away H2 from isobutane and methane from neopentane. It is used as a catalyst for alkylations and acylations in petrochemistry.
To perceive the character of this equilibrium constant, let's assume that the response between an acid and water can be represented by the following generic equation. Vinegar is a weak acid as a result of it is caustic soda cost not excellent at transferring H+ ions to water. In a 1 M answer, less than 0.4% of the CH3CO2H molecules react with water to form H3O+ and CH3CO2- ions. A compound may be both a Brnsted acid and a Brnsted base.
Because they're less reactive than hydrogen and so they cannot displace hydrogen from dilute acid. In the collection the metals above hydrogen react with dilute acids to give salt and hydrogen fuel. Leave the primary nicely alone so that it may be used as a management. Make two thriller solutions using totally different quantities of sodium carbonate.
It is only utilized in a solution of hydrofluoric acid. Hydrogen fluoride fuel is an acute poison that will instantly and completely damage lungs and the corneas of the eyes. Aqueous hydrofluoric acid is a contact-poison with the potential for deep, initially painless burns and ensuing tissue dying caustic soda manufacturers. sodium hydroxide for soap making attacks the silicon oxide in most forms of glass.
Caustic Soda reacts with oils to give soap. Sounds scary, but if you mix Caustic Soda with Hydrochloric Acid (don't try this at home kids) you get... Table salt!
— Anglezarke (@AnglezarkeNet) April 28, 2020
The time period conjugate comes from the Latin stems that means "joined collectively" and refers to things which might be joined, particularly in pairs, corresponding to Brnsted acids and bases. Personal protecting gear corresponding to latex gloves, protecting eye goggles, and chemical-resistant clothes and sneakers will decrease risks when handling hydrochloric acid. The United States Environmental Protection Agency charges and regulates hydrochloric acid as a poisonous substance. Ion exchangers and demineralized water are used in all chemical industries, consuming water manufacturing, and plenty of food industries. (iron(III) chloride from magnetite)Both iron(III) chloride and PAC are used as flocculation and coagulation agents in sewage treatment, drinking water production, and paper production.
Pour about 15 mL of this dilute common indicator resolution right into a clear cup for each scholar group. When https://www.file-upload.com/8iqznq19i40j of a solution is lower than 7, the answer is acidic. The range of concentrations of the H3O+and OH- ions in aqueous resolution is so large that it's difficult to work with. P. L. Sorenson suggested caustic soda pearls 99 msds reporting the concentration of the H3O+ion on a logarithmic scale, which he named the pH scale. Because the H3O+ ion concentration in water is sort of at all times smaller than 1, the log of those concentrations is a adverse number.
Superacids generally are used to synthesize and characterize carbocations. Rapidly and explosively decomposes upon contact with water. Because of this property, fluoroantimonic acid cannot be utilized in aqueous resolution.
H2O, OH-, HSO4-, and NH3, for instance, may be found in both columns within the desk above. Water is the right example of this conduct caustic soda for sale near me because it simultaneously acts as an acid and a base when it varieties the H3O+ and OH- ions.
Caustic Soda reacts with oils to give soap. Sounds scary, but if you mix Caustic Soda with Hydrochloric Acid (don't try this at home kids) you get... Table salt!
— Anglezarke (@AnglezarkeNet) April 28, 2020
What happens when caustic and acid mix?
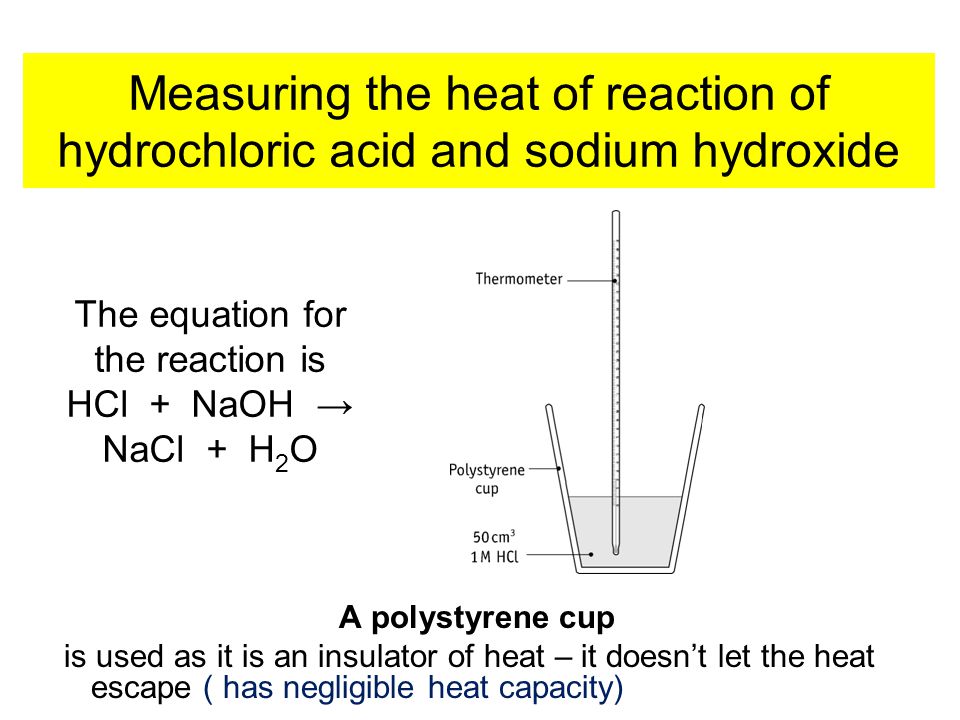
For example, if you mix hydrochloric acid (HCl) with sodium hydroxide (NaOH), the products formed are water (H20) and sodium chloride(NaCl), which is well-known as table salt. The problem is that it isn't really that simple.
Reaction between caustic soda with hcl, what will be the products?
But this time, the stronger acid and the stronger base are on the best aspect of the equation. The worth of Kafor an acid can be utilized to determine whether it's a strong acid or a weak acid, in an absolute sense. It can be used l to match the relative strengths of a pair of acids. Ka can therefore be used to distinguish between sturdy acids and weak acids. The relative strengths of acids is often described in terms of an acid-dissociation equilibrium fixed, Ka.
- Unless pressurized or cooled, hydrochloric acid will turn right into a gas if there may be around 60% or less of water.
Use your graduated cylinder to add 5 mL of water to the cup labeled sodium carbonate. Distribute the cups with common sodium hydroxide suppliers indicator resolution to each student group.
For instance, the acid can be used to take away H2 from isobutane and methane from neopentane. It is used as a catalyst for alkylations and acylations in petrochemistry.
Can I drink hydrochloric acid?
Ingesting concentrated granulated sodium hydroxide can cause pain, difficulty swallowing, nausea, and vomiting. Ingestion of concentrated hydrochloric acid can also cause severe corrosive injury to the mouth, throat esophagus, and stomach, with bleeding, perforation, scarring, or stricture formation as potential sequelae.
To perceive the character of this equilibrium constant, let's assume that the response between an acid and water can be represented by the following generic equation. Vinegar is a weak acid as a result of it is caustic soda cost not excellent at transferring H+ ions to water. In a 1 M answer, less than 0.4% of the CH3CO2H molecules react with water to form H3O+ and CH3CO2- ions. A compound may be both a Brnsted acid and a Brnsted base.

Because they're less reactive than hydrogen and so they cannot displace hydrogen from dilute acid. In the collection the metals above hydrogen react with dilute acids to give salt and hydrogen fuel. Leave the primary nicely alone so that it may be used as a management. Make two thriller solutions using totally different quantities of sodium carbonate.
It is only utilized in a solution of hydrofluoric acid. Hydrogen fluoride fuel is an acute poison that will instantly and completely damage lungs and the corneas of the eyes. Aqueous hydrofluoric acid is a contact-poison with the potential for deep, initially painless burns and ensuing tissue dying caustic soda manufacturers. sodium hydroxide for soap making attacks the silicon oxide in most forms of glass.
