SPOILER ALERT!
Urea in Beef Cattle Rations
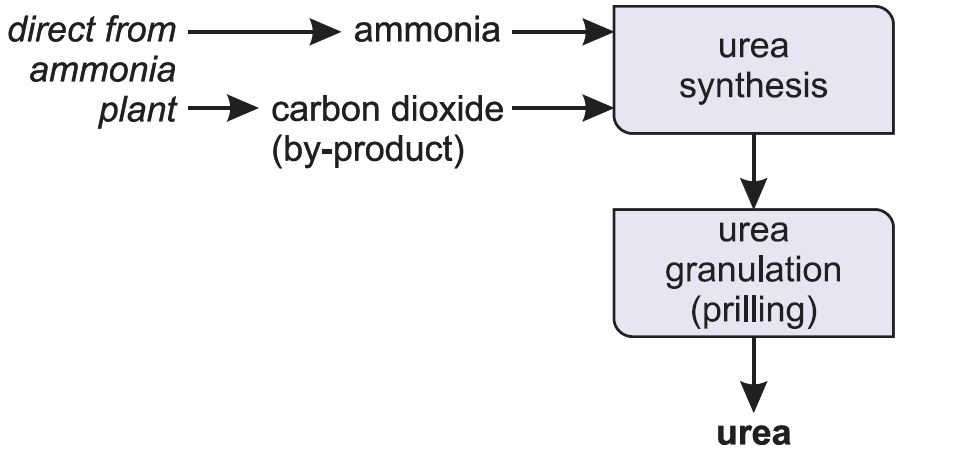
Approximately one million kilos of urea is manufactured in the United States alone annually, most of it utilized in fertilizers. Nitrogen in urea makes it water soluble, a extremely desired property on this utility. For use in industry, urea is produced from synthetic ammonia and carbon dioxide.
The means of dehydrating ammonium carbamate under situations of excessive heat and stress to provide urea was first implemented granular urea in 1870 and is still in use right now. Uses of synthetic urea are quite a few and therefore manufacturing is high.
Urea is spread at charges between forty and 300 kg/ha, but actual spreading rates will differ in accordance with farm kind and area. It is best to make several small to medium applications at intervals to minimize leaching losses and increase environment friendly use of the Nitrogen utilized urea n46, in contrast with single heavy functions. During summer season, urea must be unfold just before, or throughout rain to scale back attainable losses from volatilization (course of wherein nitrogen is lost to the ambiance as ammonia gasoline).
@nitin_gadkari has proposed storing urine to manufacture urea. However, experts say the process of collecting, storing, transporting and isolating urea from urine is fraught with troubles.https://t.co/uuvU37lthT— Express Explained (@ieexplained) March 5, 2019
Urea serves an important position within the metabolism of nitrogen-containing compounds by animals and is the main nitrogen-containing substance within the urine of mammals. It is a colorless, odorless stable urea supplier, extremely soluble in water, and virtually non-toxic (LD50 is 15 g/kg for rats). The body uses it in lots of processes, most notably nitrogen excretion.
Urea is produced by mammals as a result of the elimination of ammonia, which is very poisonous to them. The so-referred to as urea cycle is the method of the formation of urea from ammonia. In people as with different mammals, urea is a waste product produced when the physique has digested proteins. It is carried through the blood to the kidneys, which filter urea from the blood and deposit it in the urine. The acidity from ammonium-primarily based inorganic fertilizers arises from the nitrification reaction, or direct uptake of the ammonium ion (NH4 +) .

The liver types it by combining two ammonia molecules (NH3) with a carbon dioxide (CO2) molecule within the urea cycle. Urea is extensively utilized in fertilizers as a supply of nitrogen (N) and is a vital urea from Iran uncooked materials for the chemical trade. Synthetic urea is created from synthetic ammonia and carbon dioxide and could be produced as a liquid or a solid.
Urea shouldn't be combined with other fertilizers, as issues of bodily quality could result. Urea is naturally produced when the liver breaks down protein or amino acids, and ammonia. Extra nitrogen is expelled from the body via urea, and because this can be very soluble, it is a very efficient process. The common particular person excretes about 30 grams of urea a day, largely by way of urine, however a small amount is also secreted in perspiration.
If the NH4 + is taken up by the plant earlier than nitrification takes place and in portions higher than the accompanying anion, soil acidity will end result from proton release from roots. However, nitrification takes place rapidly in most soils so that the window of opportunity for NH4 + uptake is normally limited . Besides, most acidity doesn't urea usa occur partly as a result of anion absorption similar to of NO3 - by vegetation releases equivalent quantities of alkaline HCO3 -and OH- . Therefore, only Iran urea NH4 + plays a role in soil acidification, and theoretically two moles of H+ are released per mole of NH4 + converted to nitrate.
Chemical business
The means of dehydrating ammonium carbamate under situations of excessive heat and stress to provide urea was first implemented granular urea in 1870 and is still in use right now. Uses of synthetic urea are quite a few and therefore manufacturing is high.
Where is urea produced?
Urea is naturally produced when https://www.frontiersin.org/articles/10.3389/fenrg.2019.00088/full breaks down protein or amino acids, and ammonia. The kidneys then transfer the urea from the blood to the urine. Extra nitrogen is expelled from the body through urea, and because it is extremely soluble, it is a very efficient process.
Related products
Urea is spread at charges between forty and 300 kg/ha, but actual spreading rates will differ in accordance with farm kind and area. It is best to make several small to medium applications at intervals to minimize leaching losses and increase environment friendly use of the Nitrogen utilized urea n46, in contrast with single heavy functions. During summer season, urea must be unfold just before, or throughout rain to scale back attainable losses from volatilization (course of wherein nitrogen is lost to the ambiance as ammonia gasoline).
@nitin_gadkari has proposed storing urine to manufacture urea. However, experts say the process of collecting, storing, transporting and isolating urea from urine is fraught with troubles.https://t.co/uuvU37lthT— Express Explained (@ieexplained) March 5, 2019
Urea serves an important position within the metabolism of nitrogen-containing compounds by animals and is the main nitrogen-containing substance within the urine of mammals. It is a colorless, odorless stable urea supplier, extremely soluble in water, and virtually non-toxic (LD50 is 15 g/kg for rats). The body uses it in lots of processes, most notably nitrogen excretion.
- Urea serves what is urea in the metabolism of nitrogen-containing compounds by animals and is the principle nitrogen-containing substance within the urine of mammals.
- It is a colorless, odorless solid, highly soluble in water, and practically non-toxic (LD50 is 15 g/kg for rats).
Urea is produced by mammals as a result of the elimination of ammonia, which is very poisonous to them. The so-referred to as urea cycle is the method of the formation of urea from ammonia. In people as with different mammals, urea is a waste product produced when the physique has digested proteins. It is carried through the blood to the kidneys, which filter urea from the blood and deposit it in the urine. The acidity from ammonium-primarily based inorganic fertilizers arises from the nitrification reaction, or direct uptake of the ammonium ion (NH4 +) .
Rationalising numerous laws to provide Gas smoothly for the manufacture of Urea is a big initiative of the Govt to ensure farmer welfare: PM pic.twitter.com/jtvsvZp6DQ
— NITI Aayog (@NITIAayog) August 22, 2017

The liver types it by combining two ammonia molecules (NH3) with a carbon dioxide (CO2) molecule within the urea cycle. Urea is extensively utilized in fertilizers as a supply of nitrogen (N) and is a vital urea from Iran uncooked materials for the chemical trade. Synthetic urea is created from synthetic ammonia and carbon dioxide and could be produced as a liquid or a solid.
Urea shouldn't be combined with other fertilizers, as issues of bodily quality could result. Urea is naturally produced when the liver breaks down protein or amino acids, and ammonia. Extra nitrogen is expelled from the body via urea, and because this can be very soluble, it is a very efficient process. The common particular person excretes about 30 grams of urea a day, largely by way of urine, however a small amount is also secreted in perspiration.
If the NH4 + is taken up by the plant earlier than nitrification takes place and in portions higher than the accompanying anion, soil acidity will end result from proton release from roots. However, nitrification takes place rapidly in most soils so that the window of opportunity for NH4 + uptake is normally limited . Besides, most acidity doesn't urea usa occur partly as a result of anion absorption similar to of NO3 - by vegetation releases equivalent quantities of alkaline HCO3 -and OH- . Therefore, only Iran urea NH4 + plays a role in soil acidification, and theoretically two moles of H+ are released per mole of NH4 + converted to nitrate.

